Forms and Resources
Pre-Survey Packet Forms

CLIA application form, CMS-116
The official form issued by The Centers for Medicare & Medicaid Services (CMS) to apply for a CLIA certificate.

Disclosure of Ownership and Control Interest Statement- TN
The official form issued by the Tennessee CLIA State Agency (SA) to report changes in facility name, tax ID number and/or ownership

CMS-209 Laboratory Personnel Report Form
The official form issued by the Tennessee CLIA State Agency (SA) to list all laboratory personnel by their position within the laboratory setting. Included in the Pre-Survey Packet

CLIA Laboratory Specialties Test Volumes Form
The official form issued by the Tennessee CLIA State Agency (SA) to record the annual volume of all non-waived tests perform in the laboratory. Included in the Pre-Survey Packet.
Payment Instruction Forms
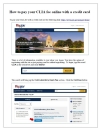
Instructions on how to use the Pay.gov system to pay your CLIA certificate fee electronically.

Intra-Governmental Payment and Collection (IPAC) System
Instructions for federal facilities to set up automated funds transfers through the IPAC payment system.
CLIA Brochures

Brochure - Verification of Performance Specifications
This brochure provides information to assist your laboratory in meeting the CLIA requirement for verifying the performance specifications of unmodified, moderate complexity tests cleared or approved by the FDA.

Brochure - Calibration and Calibration Verification
This brochure provides information to assist your laboratory in meeting the CLIA requirement of calibration and calibration verification for all nonwaived (moderate and high complexity) test systems.

Brochure - How to Obtain a CLIA Certificate
This brochure explains when a CLIA certificate is required and how to obtain one.

How to Obtain a CLIA Certficate Waiver
This brochure explains when a CLIA certificate of Waiver is required and how to obtain one.

Laboratory Director Responsibilities
What are your responsibilities as a laboratory director? This brochure explains the role and responsibilities of the laboratory director.

This brochure examines the DOs and DON’Ts of proficiency testing.

Complaints. Do You Have a Concern About a Laboratory's Operation?
This brochure explains what to do if you have a complaint about a laboratory’s operation.

What Do I Need to Do to Assess Personnel Competency?
This brochure explains the requirements for assessing the competency for laboratory personnel.

CLIA Individualized Quality Control Plan Introduction (IQCP)
This brochure provides information on CLIA’s quality control option called Individualized Quality Control Plan, also known as IQCP.

CLIA IQCP, Considerations When Deciding to Develop an IQCP
This brochure provides information on what you need to know when developing an Individualized Quality Control Plan.

This brochure provides a framework for customizing a quality control program for your test systems and your laboratory’s unique environment.

CLIA Program and Medicare Laboratory Services
This brochure provides an overview of the Clinical Laboratory Improvement Amendments (CLIA) program.
CLIA Booklets

Provider Performed Microscopy Procedures - A Focus on Quality Practices
This booklet describes recommended practices for physicians, midlevel practitioners (nurse midwife, nurse practitioner, or physician assistant), and dentists who perform patient testing under a CLIA Certificate for PPM procedures. The booklet contains an overview of the regulatory requirements, resources including forms and examples, and an overview with images of common microscopic findings for the nine specific microscopic examinations that may be performed under a Certificate of PPM Procedures.

This booklet describes recommended practices for physicians, nurses, medical assistants, pharmacists, and others who perform patient testing under a CLIA Certificate of Waiver. The booklet contains tips, reminders, and resources along with forms and examples for use in your testing site.

To Test or Not to Test? Booklet
This booklet describes considerations and preparations needed prior to performing waived testing and may assist those who want to implement and oversee waived testing or offer a new test under a CLIA Certificate of Waiver. The booklet contains tips, reminders, and resources along with forms and examples for use in your testing site.

Developing an IQCP - A Step-By-Step Guide
This workbook is designed to assist in developing an IQCP for one or more test systems. Using an example scenario, the workbook guides you through a step-by-step process to develop an IQCP that can be sustained and modified, as needed, over time. You will evaluate your current quality activities and develop an IQCP worksheet which, when completed, can serve as your IQCP document. The approach outlined in this workbook is not mandatory or the only format for documentation, but is one example that can be used.Developing an
FaxBlast

The 6 Procedures of Competency Assessment
One page flyer citing the six (6) procedures for assessment of competency for all personnel performing laboratory testing.

One page flyer announcing the start of IQCP, with link for additional information

Microbiology Media Quality Control
One page flyer with helpful cited CLIA regulations regarding microbiology media quality control.

Writing Your POC for CLIA Deficiencies
One page flyer providing tips on how to write an acceptable Plan of Correction.

One page flyer outlining helpful steps on how to prepare for a survey.

One page flyer with information on PT referral and why it matters.

One page flyer announcing a web-based training course provided by CMS. The course is designed to assist providers with the implementation of the CLIA regulatory requirement.

One page flyer announcing an increase in CLIA certificate fees, with a link to additional information.
Websites
The official website of the Clinical Laboratory Improvement Amendments (CLIA).
FDA CLIA Test Complexity Database
Access the FDA CLIA database containing the commercially marketed in vitro test systems categorized by the FDA and by the Centers for Disease Control and Prevention.

CDC CLIA Code of Federal Regulations 42 CFR 493
View the federal regulations applicable to all US clinical laboratories

How to Complete a CMS-116 Application
Instructions on how to complete a CMS-116 Application for Certification Form, needed to obtain a CLIA certificate.